Describe How the Octet Rule Applies to Covalent Bonds.
H forms one covalent bond and is generally not found as a central atom in covalent species. Introduction Within a chemical.
Chapter 4 Octet Rule And Ions Ppt Video Online Download
Describe how the octet rule applies to covalent bonds.

. Apply the octet rule to covalent compounds. Apply the octet rule d. Identify anions and cations c.
Which of the following statements correctly describe the normal bonding pattern for a neutral atom of each element assuming that the octet rule is obeyed. Describe a polar covalent bond. Illustrate the formation of single double and triple covalent bonds using Lewis structures.
The octet rule is a rule in chemistry where elements want to form bonds to attain 8 electrons in their valence shell. Draw Lewis dot structures f. How is this different from how it applies to ionicu bonds.
Describe ionic lattice structure e. Describe ionic lattice structure. Distinguish between ionic and covalent compounds II.
The shared electrons complete the octet of each atom. Problem 70 Describe a polar molecule. How is a covalent bond formed between two atoms.
Describe how the octet rule applies to covalent bonds. Explain the formation of single double and triple bonds g. Draw Lewis dot structures.
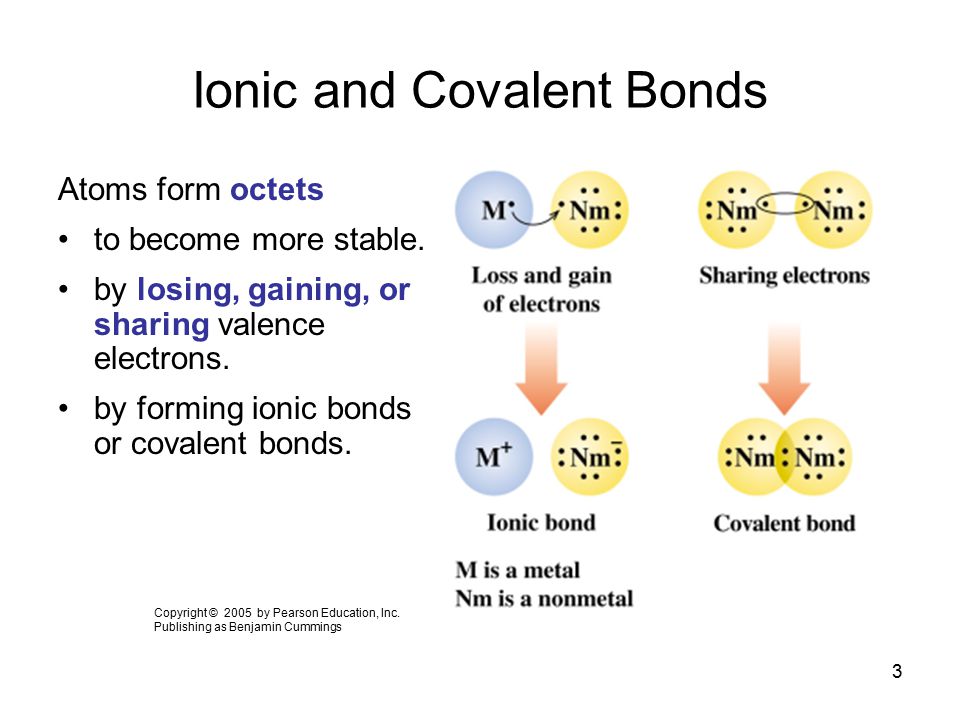
Atoms will bond by transferring ionic or sharing covalent electrons. The Octet Rule requires all atoms in a molecule to have 8 valence electrons--either by sharing losing or gaining electrons--to become stable. Atoms share valence electrons.
Ionic and Covalent Bonds. In this overview of chemical bonds learn about ionic compounds covalent bonds the octet rule Lewis structures and more. Describe how a covalent bond forms.
52 Select all that apply. Atoms share valence electrons. Describe how the octet rule applies to covalent bonds atoms shares valence electrons the shared electrons complete the octet of each atom Name the diatomic molecules.
Apply the octet rule. A similar rule applies for covalent bonds. Up to 24 cash back 1.
The octet rule states that atoms below atomic number 20 tend to combine so that they each have eight electrons in their valence shells which gives them the same electronic configuration as a noble gas. Explain the octet rule and how it applies to chemical bonding. Fforms a single covalent bond.
What are the different types of bonds and how many electrons are associated with each type of bond. It is one of an important rule in which elements of main group of perioidic table follow question_answer. Distinguish between ionic compounds and covalent compounds.
Up to 24 cash back covalent bonds. How this rule is followed when a single covalent bond a double covalent bond and a triple cova-lent bond form. Describe the formation of ionic and covalent bonds b.
What is electron sharing. Identify anions and cations. So in the case of a Covalin bond where electrons are shared that being the.
Explain the formation of single double and triple bonds. Illustrate the formation of single double and triple covalent bonds using Lewis structures. Describe how the octet rule applies to covalent bonds.
Covalent Bonding Learning Objectives To describe how a covalent bond forms. The majority of covalent bonds form between nonmetallic elements. Draw Lewis dot structures In the second part of the Ionic and Covalent Bonds simulation you will learn about the octet rule and how to apply this to building Lewis dot structures in a virtual drawing activity.
Problem 9 Illustrate the formation of single double and triple covalent bonds using Lewis structures. This gives them charges of 1 2 and 3. The octet rule states that Adams tend to gain lose or share electrons in order to achieve a full set of eight valence electrons when they form bombs.
Check all that apply. You will see that there are many ways that covalent bonds can be formed depending on the compound and electron configuration. An example of this would be sodium chloride.
Laboratory Report 21 Ionic and Covalent Bonds I. How is this different from how it applies to ionicu bonds. The octet rule states that atoms minus the exceptions want 8 electrons in their valence shell.
You can use these brief lessons and quizzes for completing a. Atoms have two ways to achieve an octet. IONIC BONDS Atoms in Groups 1 to 3 lose their electrons when they react.
- 8711915 Jewelzxc Jewelzxc 19122020 Science Senior High School answered how does the octet rule apply to covalent bonds. To apply the octet rule to covalent compounds. The majority of covalent bonds form between nonmetallic elements.
Describe the formation of ionic and covalent bonds. First lets define the octet rule and then defined Covalin bonds and see if we can describe how the octet rule applies to Covalin bonds. The octet rule causes atoms to form bonds in such a way that they acquire eight valence electrons.
The rule is applicable to the main- group elements especially carbon nitrogen oxygen and the halogens. The Octet Rule in Covalent Bonding Recall that when ionic compounds form electrons tend to be transferred so that each ion acquires a noble gas configuration. For Covalent bonds atoms tend to share their electrons with each other to satisfy the Octet RulePhosphorus needs to gain 3 electrons to fulfill the Octet Rule.
The shared electrons complete the octet of each atom. They can either lose electrons or gain electrons. The Octet Rule and Its Exceptions.
Write the corresponding number of the phrases that describe some commonexamples of. Describe how the octet rule applies to covalent bonds.

Goals 1 What Is A Covalent Bond Be Able To Describe The Nature Of Covalent Bonds And How They Are Formed 2 How Does The Octet Rule Apply To Covalent Ppt Download

Goals 1 What Is A Covalent Bond Be Able To Describe The Nature Of Covalent Bonds And How They Are Formed 2 How Does The Octet Rule Apply To Covalent Ppt Download
Comments
Post a Comment